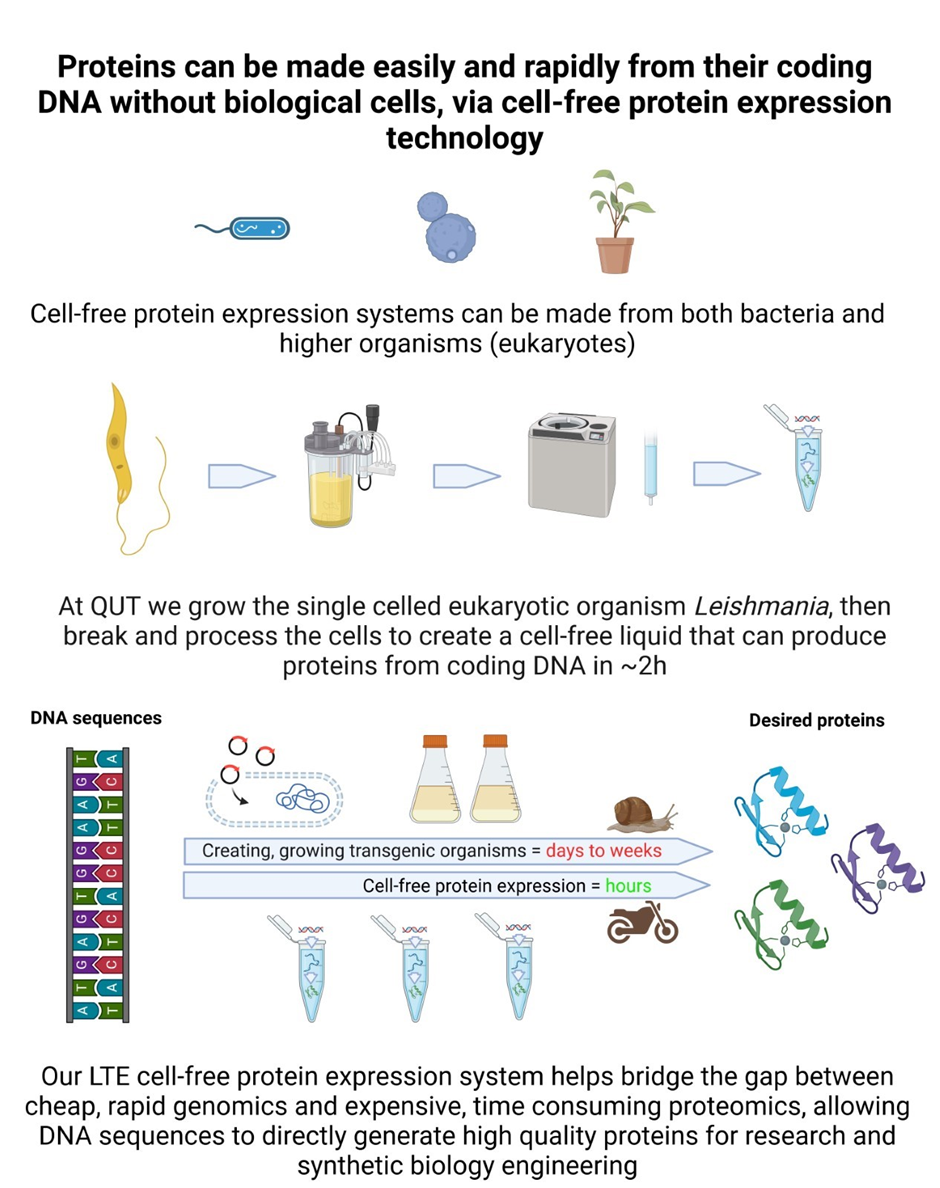
We continuously develop and apply a eukaryotic protein expression system called LTE that is based on a parasitic kinetoplastid protozoan Leishmania tarentolae. L tarentolae is easily cultivated in standard bioreactors for cell-free lysate production. A useful feature of Leishmania is its conserved endogenous mRNA leader sequence that allows suppression of all endogenous mRNA. We developed a species-independent translational sequence (SITS) that mediates efficient translation initiation in LTE and all other cell-free systems. The resulting coupled transcription-translation LTE cell-free expression system is cost-effective and simple to manufacture. Recently the LTE system has been optimized for lyophilization enabling its economical transport at room temperature and subsequent rehydration at the point of use.
LTE was used for mapping protein-protein interactions, including between SARS-CoV2 and human proteins. It has been applied to discovery of novel anti-inflammatory proteins in the hookworm secretome, and the production of enzymes for retrobiocatalysis studies.