Our ability to understand sub-cellular metabolism at the metabolite level currently relies primarily on analytical chemistry, which requires cell lysis and significant sample processing prior to analysis using chromatography and mass spectrometry. This means that there is a considerable delay between sampling and data recovery; in addition, each metabolite behaves differently during sampling and processing, leading to inaccuracies in absolute quantification relative to subcellular conditions.
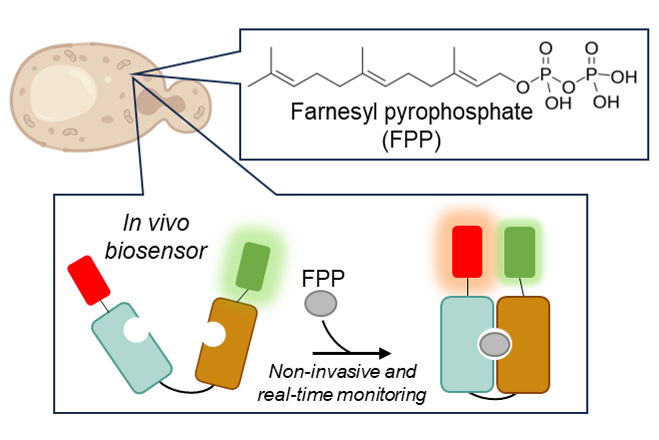
We seek to develop a minimally invasive in vivo method to read individual metabolite concentrations in real time, with high specificity and without affecting overall metabolic behaviour. We are developing protein-based biosensors with genetic response circuits that will allow us to observe changes in metabolite concentrations non-invasively in real time. These biosensors will be invaluable both for understanding subcellular metabolism and for metabolic engineering.
To prototype this tool, we are targeting farnesyl pyrophosphate (FPP). FPP is a key intermediate in the biosynthesis of many industrially valuable chemicals. We aim to develop an in vivo biosensor and gene regulatory circuit that can monitor and respond to intracellular FPP levels in yeast, enabling real-time characterization and metabolic engineering of terpene-producing yeast for industrial applications.
Project team
- Irfan Farabi Hayat
Project funding