Molecular Targeted Therapies
Our group is interested in the structure and function of cellular signaling networks, and is developing cutting-edge new mathematical approaches to map network structures to biologically important functions such as robust perfect adaptation (RPA). A key application of these new mathematical methods is identifying which proteins in these highly complex signalling networks might be sensitive to perturbation by small molecules (ie. drugs such as enzyme inhibitors) thereby suggesting novel therapeutic strategies. A broad goal is to gain a fundamental understanding of intriguing phenomena such as ‘oncogene addition’, through the development of mathematical models of signalling pathways involved in cancer, and to exploit this mathematical understanding to propose new types of targeted therapies and personalised regimens that are tailored to specific signalling aberrations.
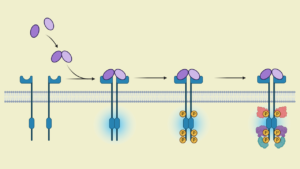
Project Titles:
- Decomposition of genome-scale metabolic chemical reaction networks into algebraically-independent subnetworks: identifying control mechanisms across evolutionary time.
- Mathematical modelling of adaptation-capable networks in the presence of enzyme-inhibitors.
- Integral control in the desensitization of G-protein coupled receptors.
- Mathematical modelling of cellular cholesterol homeostasis.
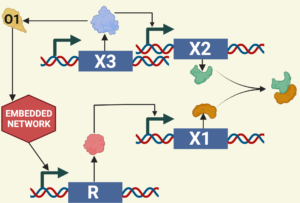
Below are some relevant publications by researchers in this domain:
- Araujo, R.P. and Liotta, L.A., 2018. The topological requirements for robust perfect adaptation in networks of any size. Nature communications, 9(1), pp.1-12.
- Araujo, R.P., Vittadello, S.T. and Stumpf, M.P., 2021. Bayesian and Algebraic Strategies to Design in Synthetic Biology. Proceedings of the IEEE.
- Araujo, R.P., Liotta, L.A. and Petricoin, E.F., 2007. Proteins, drug targets and the mechanisms they control: the simple truth about complex networks. Nature Reviews Drug Discovery, 6(11), pp.871-880.
- Petricoin, E.F., Belluco, C., Araujo, R.P. and Liotta, L.A., 2006. The blood peptidome: a higher dimension of information content for cancer biomarker discovery. Nature Reviews Cancer, 6(12), pp.961-967.
- Jeynes-Smith, C. and Araujo, R.P., 2021. Ultrasensitivity and bistability in covalent-modification cycles with positive autoregulation. Proceedings of the Royal Society A, 477(2252), p.20210069.
- Scheepers, R., Pettet, G.J., van Heijster, P. and Araujo, R.P., 2020. Cholesterol Regulation in Age-Related Macular Degeneration: A Framework for Mathematical Modelling of Drusen Biogenesis. Bulletin of Mathematical Biology, 82(10), pp.1-48.
- Petricoin III, E.F., Espina, V., Araujo, R.P., Midura, B., Yeung, C., Wan, X., Eichler, G.S., Johann Jr, D.J., Qualman, S., Tsokos, M. and Krishnan, K., 2007. Phosphoprotein pathway mapping: Akt/mammalian target of rapamycin activation is negatively associated with childhood rhabdomyosarcoma survival. Cancer research, 67(7), pp.3431-3440.
- Araujo, R.P., Doran, C., Liotta, L.A. and Petricoin, E.F., 2004. Network-targeted combination therapy: a new concept in cancer treatment. Drug Discovery Today: Therapeutic Strategies, 1(4), pp.425-433.
- Araujo, R.P., Petricoin, E.F. and Liotta, L.A., 2005. A mathematical model of combination therapy using the EGFR signaling network. Biosystems, 80(1), pp.57-69.
Current students:
- Cailan Jeynes Smith (PhD Candidate)
- Noa Levi (PhD Candidate)
- Ronel Scheepers (PhD Candidate)