Scope: Although effective treatment options are available for the majority of breast cancer patients, major unmet needs remain for treatment of metastatic disease and for treatments to prevent metastases in patients with the highly aggressive “triple negative” breast cancer (TNBC) subtype. It has recently become appreciated that immuno-suppression within the tumour microenvironment plays an important role in modulating tumour progression and metastasis. Capitalising on this new understanding, novel classes of immunotherapeutic drugs have been developed which show promise to dramatically improve treatment of patients with many types of advanced cancers. Unfortunately, these drugs showed limited efficacy in breast cancer patients, due in large part to the poor inherent immunogenicity of breast tumours often associated with an immunosuppressive microenvironment. Thus, approaches aiming to reprogram the tumour microenvironment towards a less immunosuppressive state combined with strategies enhancing tumour immunogenicity could promote the generation and deployment of immune effector responses that are crucial to achieve clinically relevant anti-tumour immune responses.
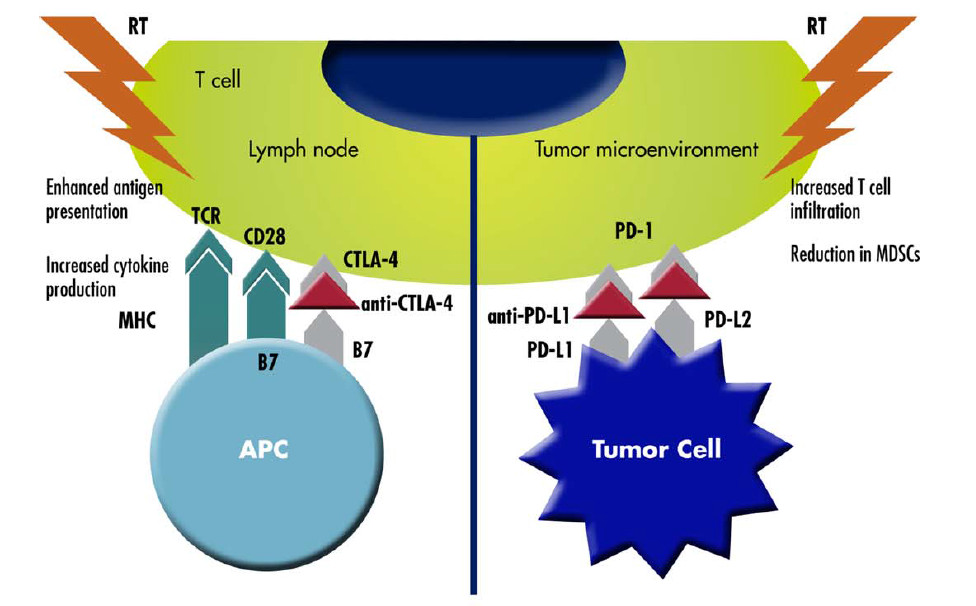
There is evidence in literature that tailored radiotherapy (RT) treatments can promote tumour immunogenicity. It is increasingly more evident that RT has several immunomodulatory effects, including induction of immunogenic cell death, increased natural killer (NK) cell activity, greater CD8+ T-cell infiltration, enhanced antigen presentation to dendritic cells, and increased production of immunostimulatory cytokines. Moreover, the understanding that the abscopal (ab, off; scopus, target) effect of RT enabling regression of non-irradiated distant metastases is mediated by the systemic immune response has escalated the interest in RT immunostimulatory activity (Salama AKS et al., Cancer. 2016).
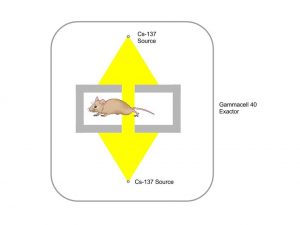
This preparatory project aims at investigating and optimising the preclinical irradiation setup. In particular, we plan to use a standard cell irradiator which is already used at the Translational Research Institute (TRI) in routine treatments of mice. The procedure followed in these cases, though, is not applicable to our project. These treatments, in fact, are total body irradiations of multiple (typically 12) mice free to move in a container. We, instead, need to perform highly conformal dose delivery, which requires precise beam collimation and accurate mice immobilization and localization. It is therefore paramount to:
- dosimetrically fully characterize the collimation system for our specific application;
- propose dosimetric quality assurance procedures for the beam energies;
- investigate what are the most appropriate mouse immobilization and localization procedures in order to have as many as possible mice irradiated at the same time, to maximize efficiency in the workflow and reduce (costly) irradiation times, while preserving irradiation efficacy (efficient irradiation of tumour tissue with minimal damage of surrounding organs and tissues).
Skills required: Multiple projects can be derived from this proposal for Masters student and/or a PhD student. Ideally the student will have a background in Medical Physics/Biomedical Engineering.
Expected output:
- A thesis
- 1-5 journal papers, depending on time-frame and scope
If you are interested in this topic and you are looking for further information, please contact me: email d3.fontanarosa@qut.edu.au, ph. 0403862724.
Check my START database entries: START
Other Team Members