The AMDTR group has a high temperature laboratory with a collection of furnaces that can be used to run high temperature exposure and electrochemical tests under any environment. These tests involved exposing alloys to the following environments:
- molten salts
- liquid sodium
- carbon dioxide
- air
- argon
- aqueous (both metals and polymers were exposed)
The main objective for these studies was to observe any degradation associated with each environment, these include degradation mechanisms such as liquid metal embrittlement, carburisation/de-carburisation and corrosion. The characterisation, detection and monitoring of these mechanisms is quintessential for many engineering applications and plays a fundamental part in the on-going maintenance of metal structures.
With a long history in conducting exposure/corrosion studies for both Concentrated Solar Power (CSP) and naval applications, the AMDTR group’s researchers have specialised expertise in this field, especially in high temperature corrosion phenomena. With access to QUT’s Central Analytical Research Facility (CARF), we have access to a variety of analysis techniques such as X-Ray Diffraction (XRD), X-Ray Fluorescence (XRF), Energy Dispersive x-ray Spectroscopy (EDS), and microscopes including an Electron Probe Micro-Analyser (EPMA) and Scanning Electron Microscope (SEM). The electrochemical experiments provide more information on the rate kinetics of corrosion reactions.
Find out more about the analysis equipment available from CARF.
Examples of molten salt exposure testing
We are able to test any salt mixture at high temperature regimes (cycling or isothermal) under any gas environment. We are able to use different testing methods to characterise the corrosion rates including thickness loss measurements, weight gain/loss measurements and cross sectional analysis using microscopy and X-ray techniques.

Typical layout of static corrosion experiment showing alloy sample exposed to a molten salt environment, cover gas can be changed from air to an inert environment using our inert gas testing system.
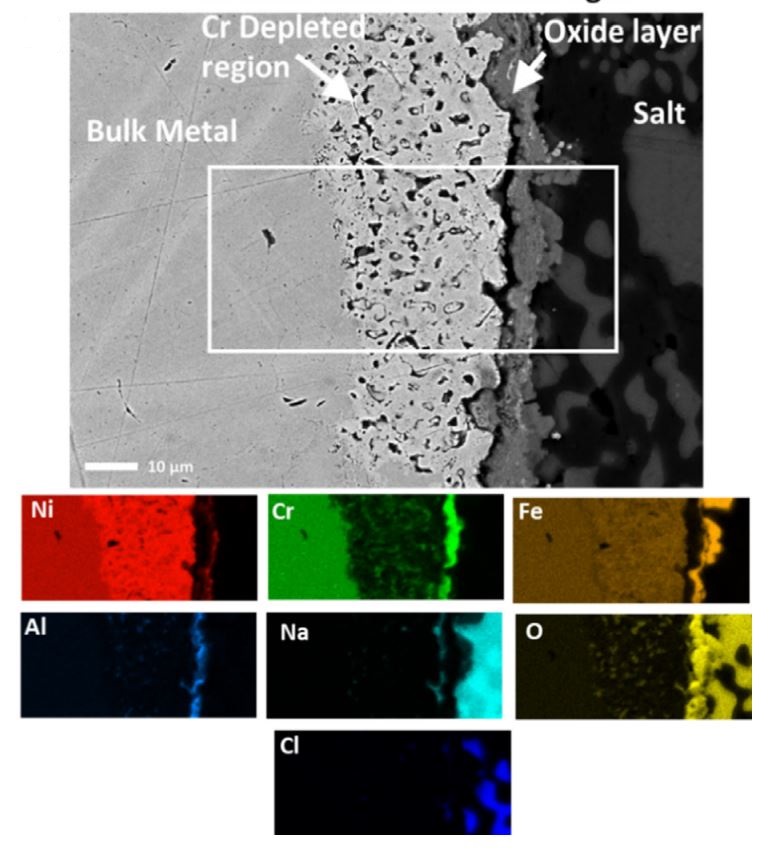
SEM backscatter cross-sectioned image of IN601 alloy sample exposed to a carbonate salt for 96 hrs isothermally, white box indicates area of where EDS mapping was undertaken. Colored EDS maps result shown underneath image. Intensity of colour indicates local concentration
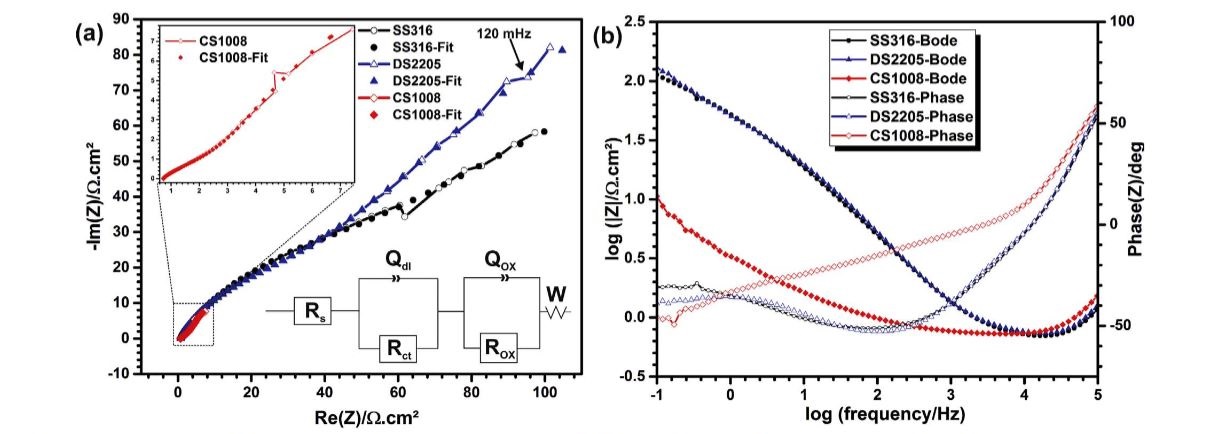
Electrochemical measurement data, (a) Nyquist and (b) Bode-Phase plot results from impedance measurements of CS1008 and, SS316 and DS2205 alloys in molten eutectic NaCl + Na2SO4 at 700 °C in air. A larger plot of CS1008, calculated equivalent circuit and fit data points are included in (a).
For examples of our molten salt corrosion studies, please read the following references:
S. Bell, M. A. Rhamdhani, T. Steinberg, and G. Will, “Aggressive corrosion of C-276 nickel superalloy in chloride/sulphate eutectic salt,” Sol. Energy, vol. 227, pp. 557–567, Oct. 2021, doi: 10.1016/j.solener.2021.09.023.
S. Bell, T. Steinberg, and G. Will, “Corrosion mechanisms in molten salt thermal energy storage for concentrating solar power,” Renew. Sustain. Energy Rev., vol. 114, p. 109328, 2019. doi: 10.1016/j.rser.2019.109328
S. Bell, K. Lippiatt, T. Steinberg, and G. Will, “Damage analysis of 601 nickel superalloy in eutectic Na2CO3/NaCl molten salt under isothermal and thermal cycling conditions,” Sol. Energy, vol. 191, pp. 637–646, Oct. 2019, doi: 10.1016/j.solener.2019.09.030.
M. Sarvghad, T. A. Steinberg, and G. Will, “Corrosion of stainless steel 316 in eutectic molten salts for thermal energy storage,” Sol. Energy, vol. 172, pp. 198–203, Sep. 2018, doi: 10.1016/j.solener.2018.03.053.
M. Sarvghad, G. Will, and T. A. Steinberg, “Corrosion of steel alloys in molten NaCl+ Na2SO4 at 700° C for thermal energy storage,” Sol. Energy Mater. Sol. Cells, vol. 179, pp. 207–216, 2018, doi: 10.1016/j.solmat.2017.11.017
M. Sarvghad, G. Will, and T. A. Steinberg, “Corrosion of Inconel 601 in molten salts for thermal energy storage,” Sol. Energy Mater. Sol. Cells, vol. 172, pp. 220–229, Dec. 2017, doi: 10.1016/j.solmat.2017.07.036.